Introduction
First-line treatment options for patients (pts) with marginal zone lymphoma (MZL) include rituximab monotherapy (R) and immunochemotherapy (IC), such as bendamustine with rituximab (BR) or RCHOP/RCVP. The efficacy of maintenance rituximab (M-R) has been studied in splenic (SMZL) and nodal (NMZL) MZL, showing a benefit in progression-free survival (PFS) when M-R is administered after BR (Rummel et al., ASCO 2018). However, the role of M-R in pts with extranodal MZL (EMZL) of the mucosa-associated lymphatic tissue (MALT), or those receiving R or RCHOP/RCVP, remains uncertain. In this study, we analyzed the outcomes of pts with MZL who received M-R after their first-line systemic therapy (R or IC) using data from a large multi-institutional retrospective cohort.
Methods
Adult pts with MZL (SMZL, NMZL, or EMZL) who were treated at 10 US medical centers from 2010 onwards were included. Pts who had received first-line treatment with either R or IC and achieved a partial (PR) or complete (CR) response to the initial therapy were divided into groups based on whether they subsequently received M-R or not. To avoid immortal-time bias, we required a minimum follow-up of 6 months from diagnosis. Outcomes included PFS, overall survival (OS) and cumulative incidence of histologic transformation (HT). Because of non-proportional hazards, Wilcoxon test was used to compare survival curves, and Cox models (augmented by multiple imputation to account for missing data) were bounded by 6 years of follow up. Multivariable models adjusted for prognostic factors including age, sex, performance status, stage, baseline hemoglobin, albumin, LDH, type of IC and CR/PR status at the end of therapy.
Results
The study included 427 pts with a median age of 63 years. Of these pts, 53% were women, and 49% had EMZL, 27% had NMZL, and 23% had SMZL. The first-line therapies received were R (54%), BR (36%), RCHOP (7%), or RCVP (3%). A CR was achieved by 70% (n=298) of pts after the initial therapy. Overall, 33% (n=140) received M-R with a median of 8 cycles. Among pts who initially had PR, 39% converted to a CR during M-R therapy. The median follow-up period was 5 years.
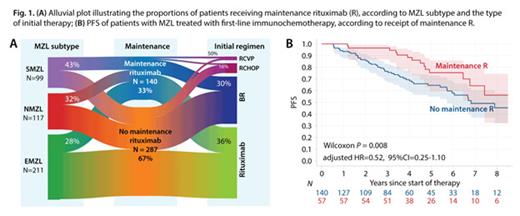
Factors associated with a higher likelihood of receiving M-R included achieving a PR instead of a CR after the first-line therapy (41% vs 29% receiving M-R, respectively; p=0.02) and having the SMZL subtype (p=0.03, Fig 1A). Pts treated with RCHOP were less likely to receive M-R compared to those receiving other regimens (p=0.049). There was also evidence of hospital-level preference to either prescribe M-R or not.
We analyzed survival outcomes separately for pts who received first-line IC or R monotherapy, stratified further by MZL subtype and the type of IC regimen. Among pts who received IC (n=197), M-R was associated with longer PFS (median of 9.0 vs. 6.8 years, p=0.008, Fig 1B). However, the statistical significance was lost in the multivariable model (adjusted HR=0.52, 95%CI=0.25-1.10). When evaluated by MZL subtype, pts with EMZL had a significantly longer PFS with M-R (p=0.01), while those with NMZL and SMZL did not (p=0.41 and 0.10, respectively). When examining the type of first-line IC, pts receiving RCHOP/RCVP had a prolonged PFS with M-R (p=0.03), whereas the difference was not statistically significant after BR (p=0.12). There was no difference in OS overall (p=0.09; adjusted HR=0.55, 95%CI=0.12-2.60) or in any subgroup.
Among pts who received first-line R, there were no statistically significant differences in PFS (p=0.70; adjusted HR=0.99, 95%CI=0.59-1.66) or OS (p=0.17) based on the receipt of M-R, either in the entire cohort or any subset.
A total of 14 transformation events occurred during the study, including 3 in the M-R group. The cumulative incidence of HT did not significantly differ between the two groups (sub-HR=0.76, 95%CI=0.21-2.82, p=0.68).
Discussion
In this large retrospective cohort study, the use of M-R in MZL was primarily influenced by local preferences, the quality of response after initial treatment, and the type of IC regimen. Receipt of M-R was associated with a longer PFS for pts receiving first-line IC, especially for those with EMZL and those receiving RCHOP/RCVP. These findings suggest a PFS advantage of M-R in EMZL, which is a novel observation. However, this advantage was not observed in terms of OS or the rate of HT. M-R after initial R did not show any significant differences in outcomes for MZL.
Disclosures
Epperla:Incyte: Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Research Funding, Speakers Bureau. Shouse:Beigene, Inc.: Speakers Bureau; Kite Pharmaceuticals: Consultancy, Speakers Bureau. Grover:Kite: Honoraria; Genentech: Honoraria; Seagen: Honoraria; Caribou Biosciences: Honoraria; Tessa Therapeutics: Research Funding; Novartis: Honoraria; Sangamo: Current holder of stock options in a privately-held company; Seattle Genetics: Consultancy; ADC Therapeutics: Consultancy, Honoraria. Torka:TG Therapeutics: Consultancy; Genmab: Consultancy; Seagen: Consultancy; Lilly USA: Consultancy; Genentech: Consultancy; ADC Therapeutics: Consultancy. Christian:F Hoffman-La Roche: Research Funding; BMS: Research Funding; Millenium: Research Funding; Acerta: Research Funding; Genentech: Research Funding. Barta:Acrotech: Consultancy; Affimed: Consultancy; Janssen: Consultancy; Daiichi Sankyo: Consultancy. Karmali:BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Miltenyi: Consultancy, Honoraria, Research Funding; Calithera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech/Roche: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Morphosys: Consultancy, Speakers Bureau; Janssen: Consultancy. Bartlett:ADC Therapeutics, Foresight Diagnostics, Kite, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics, Autolus, BMS/Celgene, Forty Seven, Gilead/Kite Pharma, Janssen, Merck, Millennium, Pharmacyclics, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Research Funding; Washington University School of Medicine: Current Employment. Olszewski:Leukemia & Lymphoma Society, Genetech, Inc. / F. Hoffmann-La Roche Ltd, Adaptive Biotechnologies, Precision Biosciences, Genmab: Research Funding; Genmab, Blue Cross/Blue Shield of Rhode Island, Schrodinger, ADC Therapeutics, BeiGene: Consultancy.